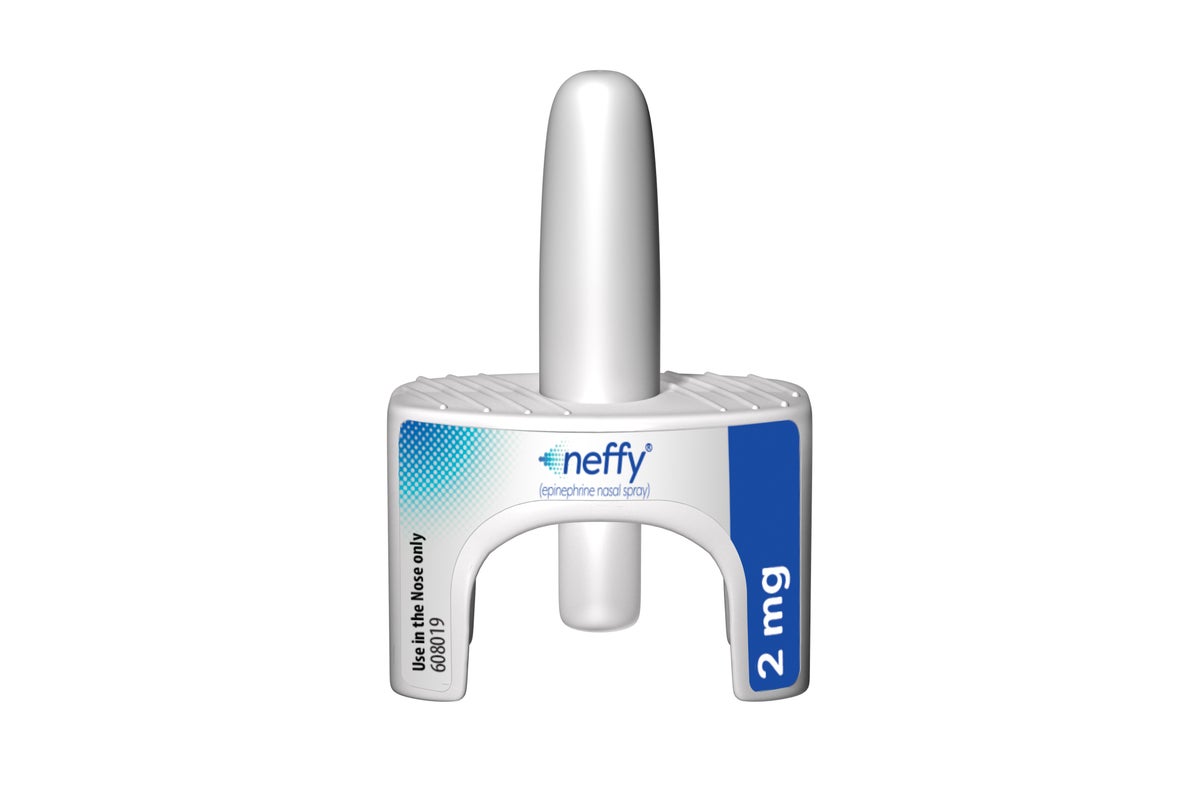
U.S. health officials approve first nasal spray for severe allergies
U.S. health officials on Friday approved the first nasal spray to treat severe allergic reactions, offering an alternative to injectable products like EpiPen. The Food and Drug Administration said it approved the spray from drugmaker ARS Pharmaceuticals Inc. as an emergency treatment for adults and older children experiencing life-threatening allergic reactions known as anaphylaxis. The spray is intended for people who weigh at least 66 pounds.
Anaphylaxis occurs when the body’s immune system develops a sudden, unexpected reaction to a foreign substance, such as food, insect stings or medications. Common symptoms include hives, swelling, itching, vomiting and difficulty breathing.
The spray, which will be sold as Neffy, is one of several needle-free devices being developed to treat between 33 million and 45 million Americans with severe allergies to food and other triggers. Neffy is given in a single dose sprayed into one nostril.
The Associated Press Health and Science Department receives support from the Howard Hughes Medical Institute’s Science and Educational Media Group. The AP is solely responsible for all content.